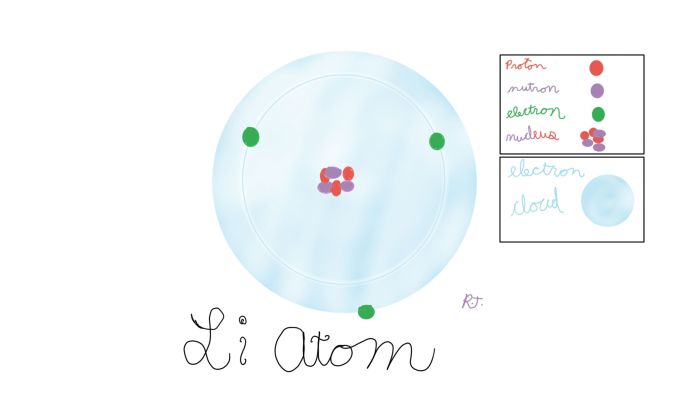
 What is an atom? Atoms are the building blocks of the universe. These building blocks are so microscopic that you need a Scanning Tunneling Microscope to see them. For more information watch this video. Atoms are made up of protons, neutrons, and electrons. The neutrons and protons made up a nucleus inside the electron shell. The electron shell is the electrons moving around in a circle around the nucleus so fast it makes a cloud. Each element has a different number of protons. The atomic number in each element box of the periodic table shows the number of protons. Normally the electrons match the protons in number, but sometimes the electrons are a different number. If you could change the number of protons it would become a new element. Many scientists have tried to create gold but have not succeeded.
What is an atom? Atoms are the building blocks of the universe. These building blocks are so microscopic that you need a Scanning Tunneling Microscope to see them. For more information watch this video. Atoms are made up of protons, neutrons, and electrons. The neutrons and protons made up a nucleus inside the electron shell. The electron shell is the electrons moving around in a circle around the nucleus so fast it makes a cloud. Each element has a different number of protons. The atomic number in each element box of the periodic table shows the number of protons. Normally the electrons match the protons in number, but sometimes the electrons are a different number. If you could change the number of protons it would become a new element. Many scientists have tried to create gold but have not succeeded.
Have you ever wondered how they organized the periodic table and by what did they classify everything? Scientists have classified them by the number of protons they have which is as I said earlier the atomic number. Hydrogen is 1, Helium is 2, and Lithium is 3, until 118. That is the number of elements on the periodic table. The 4 most recent were just announced in December. Their atomic numbers are 113, 115, 117, & 118.
On the periodic table (as shown in the previous post) there are rows and columns of boxes. These rows and columns have different names. Instead of columns, we say group, and instead of row we say period. So the periodic table is made up of groups and periods. In each group, the elements have some similarities.
I will by studying these elements by their groups, not periods, to catch the similarities. My next post will be about group 1, the Alkali Metals.
Interesting. I never knew that the number of protons define an element (at least if I did know it, I forgot). What elements don’t have an equal amount of protons to electrons? If you remove an electron from an element, does that change the characteristics of that element?
LikeLike
I don’t know, Dad. I’ll have to study it more.
LikeLike